In a major leap forward for cancer care, the BREAKWATER trial has revealed groundbreaking results that could change how doctors treat a specific type of colorectal cancer. This large-scale study, focused on patients with metastatic colorectal cancer (mCRC) harboring a BRAF V600E mutation, shows that a new combination therapy significantly improves survival rates compared to traditional treatments. The findings, shared at the 2025 ASCO Annual Meeting and published in the New England Journal of Medicine, mark a turning point for patients facing this aggressive form of cancer.
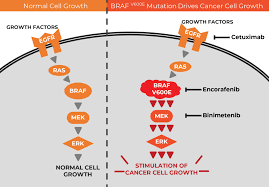
Understanding BRAF V600E Colorectal Cancer
Colorectal cancer, which affects the colon or rectum, is one of the most common cancers worldwide. Within this group, about 8% to 12% of patients with metastatic colorectal cancer have a BRAF V600E mutation. This genetic change makes the cancer more aggressive, leading to faster tumor growth and a poorer prognosis. Patients with this mutation often face a risk of death more than twice as high as those without it. Until recently, treatment options for these patients were limited, and standard chemotherapy often fell short in controlling the disease.
The BREAKWATER trial, led by researchers from The University of Texas MD Anderson Cancer Center, aimed to address this gap. The study tested a new approach combining three treatments: encorafenib (sold as Braftovi), cetuximab (sold as Erbitux), and mFOLFOX6, a chemotherapy regimen that includes fluorouracil, leucovorin, and oxaliplatin. This combination was compared to standard treatments, such as chemotherapy with or without bevacizumab, to see if it could offer better outcomes.
Key Findings from the BREAKWATER Trial
The BREAKWATER trial was a Phase 3, randomized, open-label study involving 637 patients with previously untreated BRAF V600E-mutated metastatic colorectal cancer. The results were striking. Patients who received the combination of encorafenib, cetuximab, and mFOLFOX6 saw their median overall survival (OS) nearly double, reaching 30.3 months compared to 15.1 months for those on standard therapy. This means patients lived, on average, over a year longer with the new treatment.
The trial also showed a 47% reduction in the risk of disease progression or death, with progression-free survival (PFS) extending to 12.8 months compared to 7.1 months for the control group. Additionally, the overall response rate (ORR)—the percentage of patients whose tumors shrank or disappeared—was significantly higher with the new regimen, at 60.9% compared to 40% for standard chemotherapy. These numbers highlight not only longer survival but also better control over the cancer’s growth.
Why These Results Matter
For patients with BRAF V600E-mutated colorectal cancer, these findings are a beacon of hope. Historically, this group has faced limited treatment options and poor outcomes. The aggressive nature of the mutation often leads to rapid cancer progression, making effective first-line treatments critical. The BREAKWATER trial shows that combining targeted therapies like encorafenib, which blocks the BRAF mutation, and cetuximab, which targets the EGFR protein, with chemotherapy can tackle the cancer more effectively than traditional approaches.
This combination works by attacking the cancer from multiple angles. Encorafenib stops the BRAF V600E mutation from driving tumor growth, while cetuximab blocks signals that help cancer cells survive. The mFOLFOX6 chemotherapy adds another layer, directly killing cancer cells. Together, they create a powerful synergy that outperforms standard treatments, offering patients both longer lives and better quality of life.

A Step Toward Precision Medicine
The success of the BREAKWATER trial underscores the growing importance of precision medicine in cancer treatment. Precision medicine tailors therapies to the specific genetic makeup of a patient’s tumor, rather than using a one-size-fits-all approach. By focusing on the BRAF V600E mutation, the trial shows how understanding a tumor’s molecular profile can lead to better outcomes. Experts like Scott Kopetz, a lead researcher in the study, emphasize that these results could set a new standard of care for patients with this mutation.
The trial’s findings also build on earlier research. In December 2024, the U.S. Food and Drug Administration (FDA) granted accelerated approval to the encorafenib, cetuximab, and mFOLFOX6 combination based on its strong response rates. The latest survival data from BREAKWATER strengthens the case for full FDA approval, potentially making this regimen the go-to first-line treatment for BRAF V600E-mutated colorectal cancer.
What This Means for Patients
For patients diagnosed with BRAF V600E-mutated metastatic colorectal cancer, the BREAKWATER trial offers new possibilities. Doctors may now recommend early molecular testing to identify this mutation, ensuring patients get the most effective treatment from the start. Arvind Dasari, an associate professor at MD Anderson, noted that this combination should be considered the new standard for first-line treatment in these patients.
The treatment’s safety profile is also encouraging. While serious side effects were slightly higher in the combination group (46.1% compared to 38.9% in the control group), they were manageable and consistent with what doctors expect from these drugs. Common side effects included skin reactions, fatigue, and digestive issues, but no unexpected safety concerns emerged.
A Broader Impact on Cancer Care
The BREAKWATER trial’s success goes beyond colorectal cancer. It highlights the power of combining targeted therapies with chemotherapy, a strategy that could inspire new treatments for other cancers with similar genetic mutations. Researchers are already exploring how these findings might apply to other tumor types, potentially broadening the impact of this approach.
Pfizer, the maker of encorafenib, is working with the FDA and other global health authorities to secure full approval for this regimen. Roger Dansey, Pfizer’s interim chief oncology officer, called the results “practice-changing,” noting that they address a critical need for patients with limited options. The trial’s data, presented at major conferences like ASCO, is sparking conversations among oncologists about rethinking treatment strategies for this challenging cancer.
Looking Ahead
While the BREAKWATER trial is a significant step forward, there’s still work to be done. Researchers are continuing to analyze data from the study, including results from a separate arm testing encorafenib and cetuximab without chemotherapy. These findings could provide more options for patients who may not tolerate chemotherapy. Additionally, ongoing studies are exploring how to make treatments even more effective, possibly by combining them with immunotherapies or other novel therapies.
For patients and families affected by BRAF V600E-mutated colorectal cancer, the BREAKWATER trial offers a renewed sense of optimism. It’s a reminder that science is making strides against even the toughest cancers, one breakthrough at a time. As more data emerges and treatments evolve, the hope is that more patients will live longer, healthier lives.
Conclusion
The BREAKWATER trial has set a new benchmark for treating BRAF V600E-mutated metastatic colorectal cancer. By combining encorafenib, cetuximab, and mFOLFOX6, this regimen nearly doubles survival time and significantly reduces the risk of disease progression. These results, backed by rigorous research and presented at major medical conferences, signal a shift toward more personalized, effective cancer care. For patients facing this aggressive form of cancer, the trial’s findings are a lifeline, offering better outcomes and a brighter future. As the medical community continues to build on this success, the hope is that more lives will be saved through innovative, targeted treatments.
Must Read :- The 2026 Jeep Cherokee: A Bold Comeback for an American Icon






