In recent years, gene-editing technology has taken the medical world by storm, Treatment offering new hope for treating some of the most challenging diseases, including cancer. Clinical trials using gene-editing tools like CRISPR are showing promising results, and the U.S. Food and Drug Administration (FDA) is fast-tracking approvals for these innovative therapies. While the potential to save lives is exciting, these advancements are also sparking important ethical debates about the implications of altering the human genome. This article explores the progress of gene-editing trials for cancer treatment, the FDA’s role in speeding up approvals, and the ethical questions that come with genetic modifications.

A New Era in Cancer Treatment
Cancer remains one of the leading causes of death worldwide, with millions diagnosed each year. Traditional treatments like chemotherapy, radiation, and surgery often come with harsh side effects and varying success rates. Gene-editing technologies, particularly CRISPR, are changing the game by targeting the root causes of cancer at the genetic level.
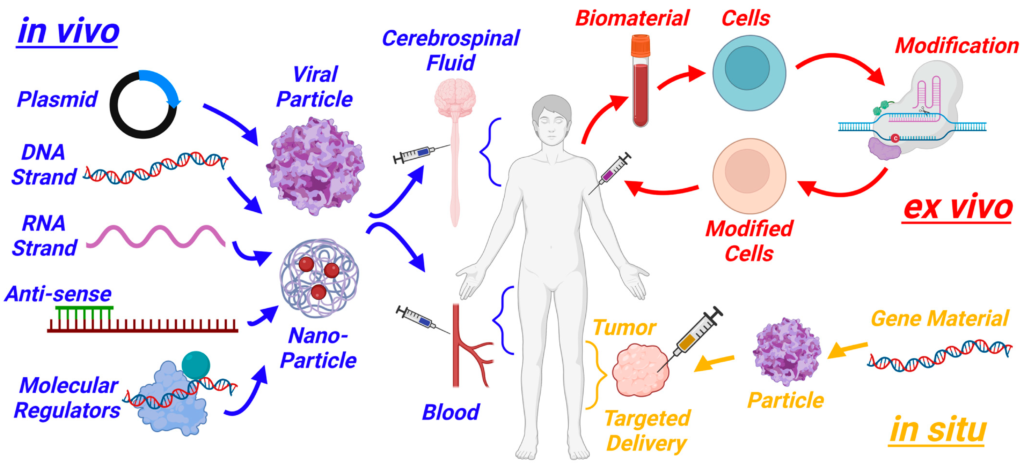
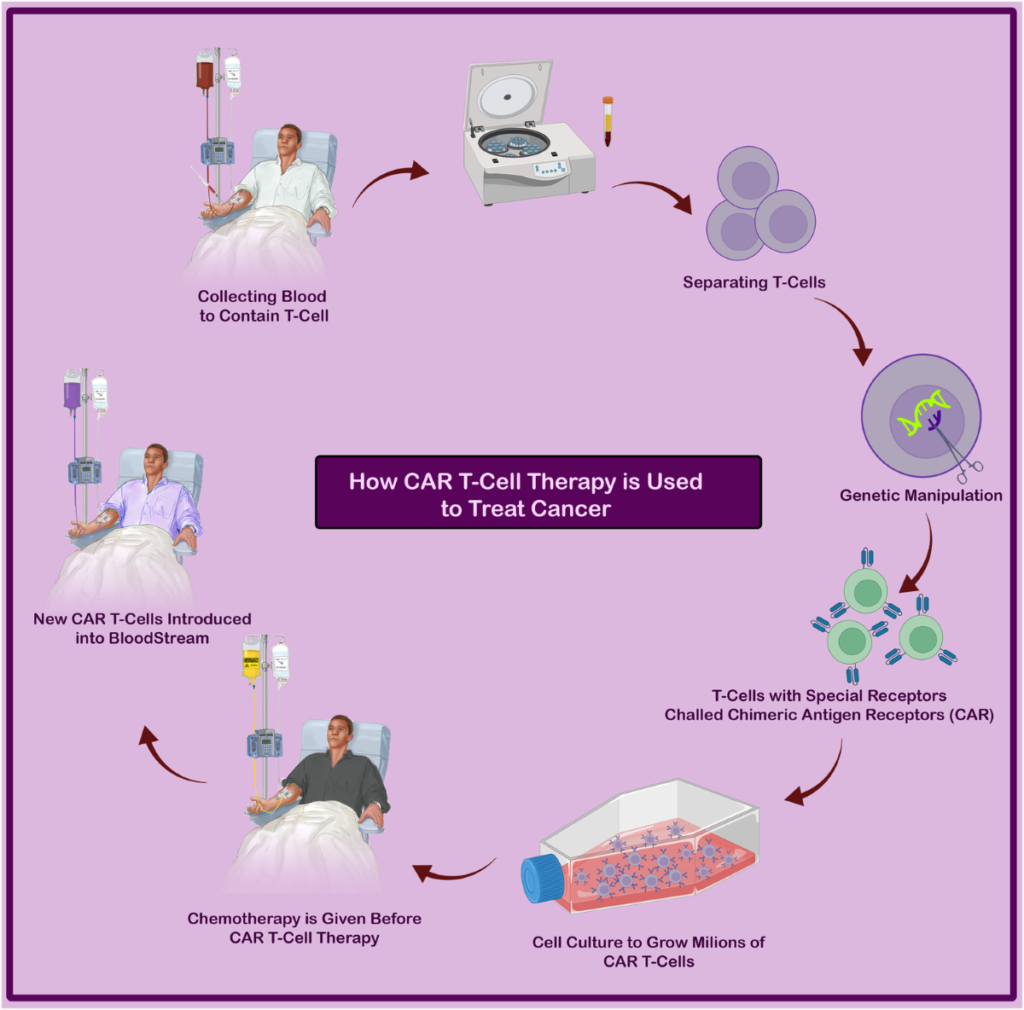
CRISPR, short for Clustered Regularly Interspaced Short Palindromic Repeats, acts like molecular scissors, allowing scientists to cut, edit, or replace specific parts of DNA. In cancer treatment, this technology is being used to modify immune cells, such as T-cells, to better recognize and attack cancer cells. For example, CAR-T cell therapy, a type of gene-edited treatment, involves taking a patient’s T-cells, genetically modifying them to target cancer cells, and reintroducing them into the body. This approach has shown remarkable success in treating blood cancers like leukemia and lymphoma.
Recent trials have expanded the use of gene-editing to solid tumors, which are harder to treat. In 2024, a trial for a rare sarcoma saw the FDA approve a genetically modified T-cell therapy, marking a significant step forward. Early results from these trials show that patients are experiencing tumor shrinkage and, in some cases, complete remission. For many, this feels like a miracle—a chance to beat a disease that once seemed unbeatable.
The FDA’s Fast-Track Push
The FDA has recognized the potential of gene-editing therapies and is working to bring them to patients faster. Through programs like Fast Track, Breakthrough Therapy, and Accelerated Approval, the agency is speeding up the review process for treatments that address unmet medical needs. These programs allow promising therapies to move through clinical trials and reach the market more quickly, giving patients access to cutting-edge treatments sooner.
For example, in December 2023, the FDA approved Casgevy, a CRISPR-based therapy for sickle cell disease, which also has applications for cancer-related conditions like beta-thalassemia. This approval was a landmark moment, as it marked the first CRISPR therapy to gain regulatory clearance in the U.S. The FDA’s willingness to fast-track these therapies reflects the urgent need for new cancer treatments and the confidence in gene-editing’s potential.
However, the fast-track process isn’t without risks. Speeding up approvals can mean less time to study long-term effects, raising concerns about safety. The FDA requires rigorous testing, but some worry that the push for rapid approvals could overlook rare side effects or complications that only appear years later. Despite these concerns, the agency maintains that patient safety remains a top priority, with strict oversight throughout the trial process.
Ethical Questions Surrounding Genetic Modifications
While the promise of gene-editing is undeniable, it comes with a host of ethical challenges. Altering the human genome raises questions about safety, equality, and the potential for misuse. Here are some of the key ethical concerns:
Safety and Long-Term Risks
One of the biggest worries is the safety of gene-editing. CRISPR is precise, but it’s not perfect. Off-target edits—changes to unintended parts of the DNA—could lead to serious health problems, including new genetic disorders or even cancer. Mosaicism, where some cells carry the edit while others don’t, is another concern, as it could result in unpredictable outcomes. Researchers are working to improve the accuracy of gene-editing tools, but the technology is still young, and long-term risks are not fully understood.
The FDA and other regulatory bodies require long-term monitoring of patients who receive gene-edited therapies to catch any delayed complications.
Know More :- 9 Cybersecurity Trends Every US Business Must Know Now






